Abstract
INTRODUCTION
There is a growing interest in analyzing the biological and clinical variables that might help in identifying patients at risk of histological transformation (HT), a critical event that can still lead to reduced survival in patients with follicular lymphoma (FL). As part of the Aristotle study, we have previously reported that the risk of HT as a first event has been significantly reduced by the use of rituximab (Federico et al, Lancet Hematology 2018). However, this incidence might still be unacceptable in some groups of patients. Thus, we investigated the risk of HT in patients with primary refractory FL, a subset of patients with poor prognosis.
METHODS
We carried out a retrospective analysis of cumulative incidence of HT in the 289 cases with primary refractory FL retrieved from the Aristotle Study, which included a total of 6970 FL cases (grade 1, 2 or 3a), diagnosed between 1997 and 2013 and treated with systemic therapy. Refractoriness was defined as progressive disease (PD) within the end of first-line therapy. Patients with PD were compared to patients with partial response (PR) or stable disease (SD) and to patients with complete response (CR) at the end of therapy. The cumulative incidence of HT was calculated using the Nelson-Aalen estimator, with a 95 confidence interval (CI). The estimate is in absolute value, and then multiplied by 100. The overall survival (OS) and survival after relapse/progression (SAR) were calculated using Kaplan-Meier estimation, with a 95% CI.
RESULTS
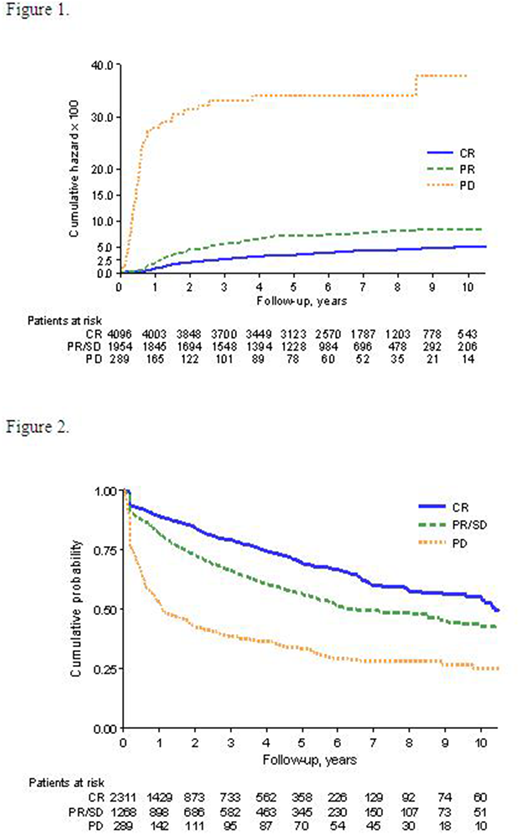
Six hundred and twenty-eight cases (9%) were excluded for incomplete data and 6,339 were considered for analysis. The five-year cumulative incidence of HT among patients with PD was 34.2% (95% CI: 26.9-43.4), whilst it was 7.1% (95% CI: 6.0-8.5) and 3.5% (95% CI: 3.0-4.2) in the 1,954 and 4,096 patients with PR+SD and CR at the end of induction therapy, respectively. The rate observed in PD group had a Hazard Ratio (HR) of 11.4 (95CI: 8.61-15.0) compared with CR patients, and 6.14 (95% CI: 4.6-8.2), when compared with PR+SD patients. All comparisons and estimations showed a p-value below 0.001. Cumulative incidences of HT according to response to initial therapy are represented in Figure 1.
The five-year SAR was 33% (95% CI: 28-39) for the PD group, with a median SAR of 1.1 year (95% CI: 0.8-1.8). At the same time point, the SAR was 56% (95% CI: 53-60) in patients with initial PR/SD, with a median SAR of 6.3 years (95% CI: 5.7-8.6). Finally, in patients with initial CR who later relapsed, the 5 year SAR was 63% (95% CI: 66-72), with a median of 10.4 years (95% CI: 10-12.2). The difference among groups was statistically significant (p-value <0.001, Figure 2). HR for PD group was 3.39 (95% CI: 2.87-4.01) compared to CR, and 2.12 (95% CI: 1.8-2.5) compared to PR/SD group.
CONCLUSIONS
According to the results of the present study, primary refractory FL patients have not only an already known reduced SAR (Casulo et al, JCO 2015), but also a dramatically increased risk of HT, which probably contributes to patients' adverse outcome. These findings reinforce the need to develop combined diagnostic and therapeutic strategies aimed to eradicate the disease at the "first shot", reducing the risk of refractoriness and eventually further improving the outcome of FL patients.
Sarkozy:ROCHE: Consultancy. Wondergem:NOVARTIS: Other: advisory board, educational talk; SANOFI: Other: advisory board; GILEAD: Other: educational talk. Chamuleau:Genmab: Research Funding; BMS: Research Funding; celgene: Research Funding; Gilead: Research Funding. Gomes da Silva:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: lecture fees, Institution's payment for consultancy, Travelling support; Celgene: Other: Travelling support; Roche: Other: Institution's payment for consultancy, Travelling support; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: lecture fees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: lecture fees; Gilead Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: lecture fees, Research Funding. Holte:Roche, Norway: Research Funding; Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees. Zucca:CELGENE: Other: Grant to the Institution + Advisory Board; Janssen: Other: Grant to the Institution; ROCHE: Other: Grant to the Institution + Advisory Board; Celltrion Healthcare + Mei Pharma + Astra Zeneca: Other: Advisory Board; AbbVie + Gilead: Other: Travel Expenses; Gilead + Bristol-Myers Squibb + MDS: Other: Expert Statement + Meetings. Aurer:Roche: Consultancy, Honoraria, Research Funding; Pfizer: Honoraria, Research Funding. Marcheselli:Fondazione Italiana Linfomi (FIL) Onlus: Employment. Salles:Novartis: Consultancy, Honoraria; Acerta: Honoraria; Abbvie: Honoraria; Morphosys: Honoraria; Epizyme: Honoraria; Amgen: Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Celgene: Honoraria, Other: Advisory Board, Research Funding; Pfizer: Honoraria; Gilead: Honoraria, Other: Advisory Board; Janssen: Honoraria, Other: Advisory Board; Merck: Honoraria; Servier: Honoraria, Other: Advisory Board; Takeda: Honoraria; BMS: Honoraria, Other: Advisory Board; Servier: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal